Berkelium
97
Bk
Gruppe
n/a
Periode
7
Blok
f
Protoner
Elektroner
Neutroner
97
97
150
Generelle Egenskaber
Atomnummer
97
Atommasse
[247]
Masseantal
247
Kategori
Actinider
Farve
n/a
Radioaktiv
Ja
Named after Berkeley, California, the city of its discovery
Krystalstruktur
Simpel Hexagonal
Historie
Berkelium was discovered by Glenn T. Seaborg, Albert Ghiorso and Stanley G. Thompson in 1949 at the University of California, Berkeley.
It was produced by the bombardment of americium with alpha particles.
Berkelium was isolated in greater quantities for the first time by Burris Cunningham and Stanley Thompson in 1958.
It was produced by the bombardment of americium with alpha particles.
Berkelium was isolated in greater quantities for the first time by Burris Cunningham and Stanley Thompson in 1958.
Elektroner i hver skal
2, 8, 18, 32, 27, 8, 2
Elektronkonfiguration
[Rn] 5f9 7s2
Just over one gram of berkelium has been produced in the United States since 1967
Fysiske Egenskaber
Tilstandsform
Fast stof
Massefylde
14,78 g/cm3
Smeltepunkt
1259,15 K | 986 °C | 1806,8 °F
Kogepunkt
3173,15 K | 2900 °C | 5252 °F
Smeltevarme
n/a
Fordampningsvarme
n/a
Varmefylde
-
Forekomst i jordskorpen
n/a
Forekomst i universet
n/a
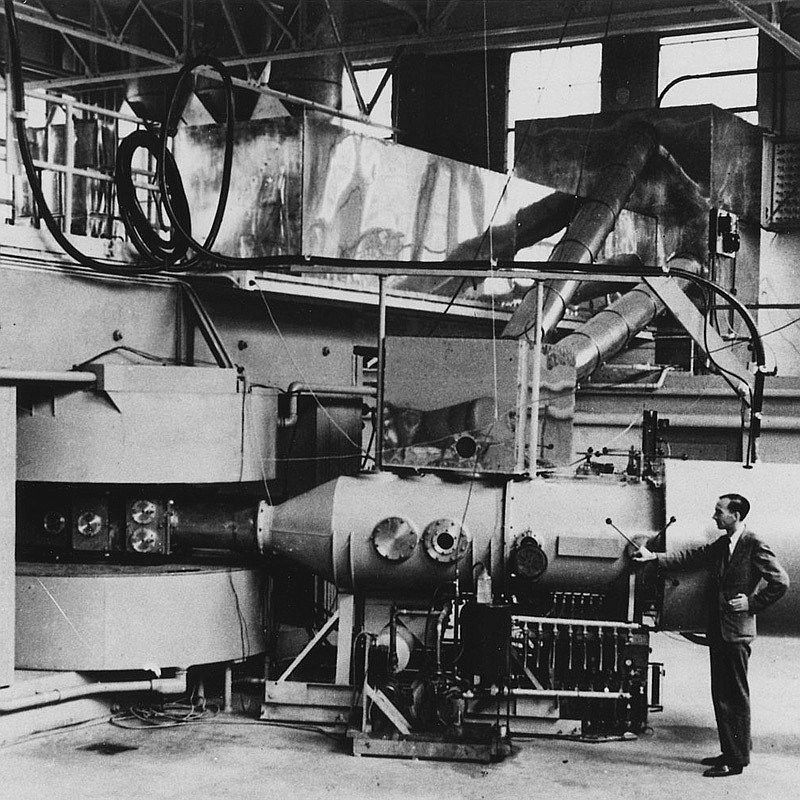
Billede akkrediteringer: Wikimedia Commons (Department of Energy - Office of Public Affairs)
The 60-inch cyclotron at the Lawrence Radiation Laboratory, University of California, Berkeley
CAS-nummer
7440-40-6
PubChem CID-nummer
23971
Atomare egenskaber
Atomradius
170 pm
Kovalent radius
-
Elektronegativitet
1,3 (Paulings skala)
Ioniseringspotentiale
6,1979 eV
Atomvolumen
16,7 cm3/mol
Varmeledningsevne
0,1 W/cm·K
Oxidationstrin
3, 4
Anvendelser
Berkelium is mainly used for scientific research purposes.
Berkelium-249 is a common target nuclide to prepare still heavier transuranic elements and transactinides, such as lawrencium, rutherfordium and bohrium.
It is also useful as a source of the isotope californium-249.
Berkelium-249 is a common target nuclide to prepare still heavier transuranic elements and transactinides, such as lawrencium, rutherfordium and bohrium.
It is also useful as a source of the isotope californium-249.
Berkelium is harmful due to its radioactivity
Isotoper
Stabile isotoper
-Ustabile isotoper
233Bk, 235Bk, 236Bk, 237Bk, 238Bk, 239Bk, 240Bk, 241Bk, 242Bk, 243Bk, 244Bk, 245Bk, 246Bk, 247Bk, 248Bk, 249Bk, 250Bk, 251Bk, 252Bk, 253Bk, 254Bk